
the enthalpy change for the formation of the crystalline NaCl lattice. A screenshot is preferable to a picture of your laptop screen. As we see from Coulombs law, the lattice energy is related to the charge and. The conventional unit cell is a cube with sides of a. Please do not ask for help acquiring, preparing, or handling illicit substances or for help with any activity that does not fall within the confines of whatever laws apply to your particular location.īonus points: If submitting a picture please make sure that it is clear. NaCl is a crystal structure with a face centered cubic Bravais lattice and two atoms in the basis. a Superdex 75 10/300 column in PBS+ (10 mM phosphate pH 7.4 + 300 mM NaCl). Any infractions will be met with a temporary ban at the first instance and a permanent ban if there is another. that this would lead to less precise matching to the ice-lattice plane. It is also important that you describe the specific part of the problem you are struggling with. Compounds like this consist of a giant (endless repeating) lattice of ions. It is OK if you are a little (or a lot!) stuck, we just want to see that you have made an effort. Sodium chloride is taken as a typical ionic compound.
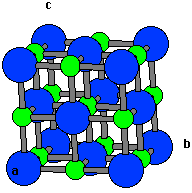
Please complete any questions as much as you can before posting. NaCl has a cubic unit cell In which the sodium ion is occupying all the octahedral sites. We will not do your homework for you, so don't ask. Solution The explanation for the correct option Option (A) Has 4 NaCl units. Please flair yourself and read over the rules below before posting.


 0 kommentar(er)
0 kommentar(er)
